Why extinguish with foam?
Fire can only exist in the presence of a combination of fuel, oxygen, and a sufficiently high temperature. Extinguishing foam is a highly effective extinguishing agent due to the following properties:
Separating action - A closed foam blanket separates the fuel from the oxygen.
Cooling action – The water in the foam evaporates. This draws heat out of the fire.
Vapour suppression - A closed foam blanket prevents further formation of gas vapours from the fuel.
Displacing effect - Because foam accumulates in small spaces, oxygen is displaced from them.
Insulating effect - The low thermal conductivity of foam insulates any fuel that has not yet ignited and protects it against the heat.
Film formation - One of the most important properties of extinguishing foams is the formation of a layer of film on the fuel. As a result, the fuel is shut off from the oxygen and ignition becomes impossible.
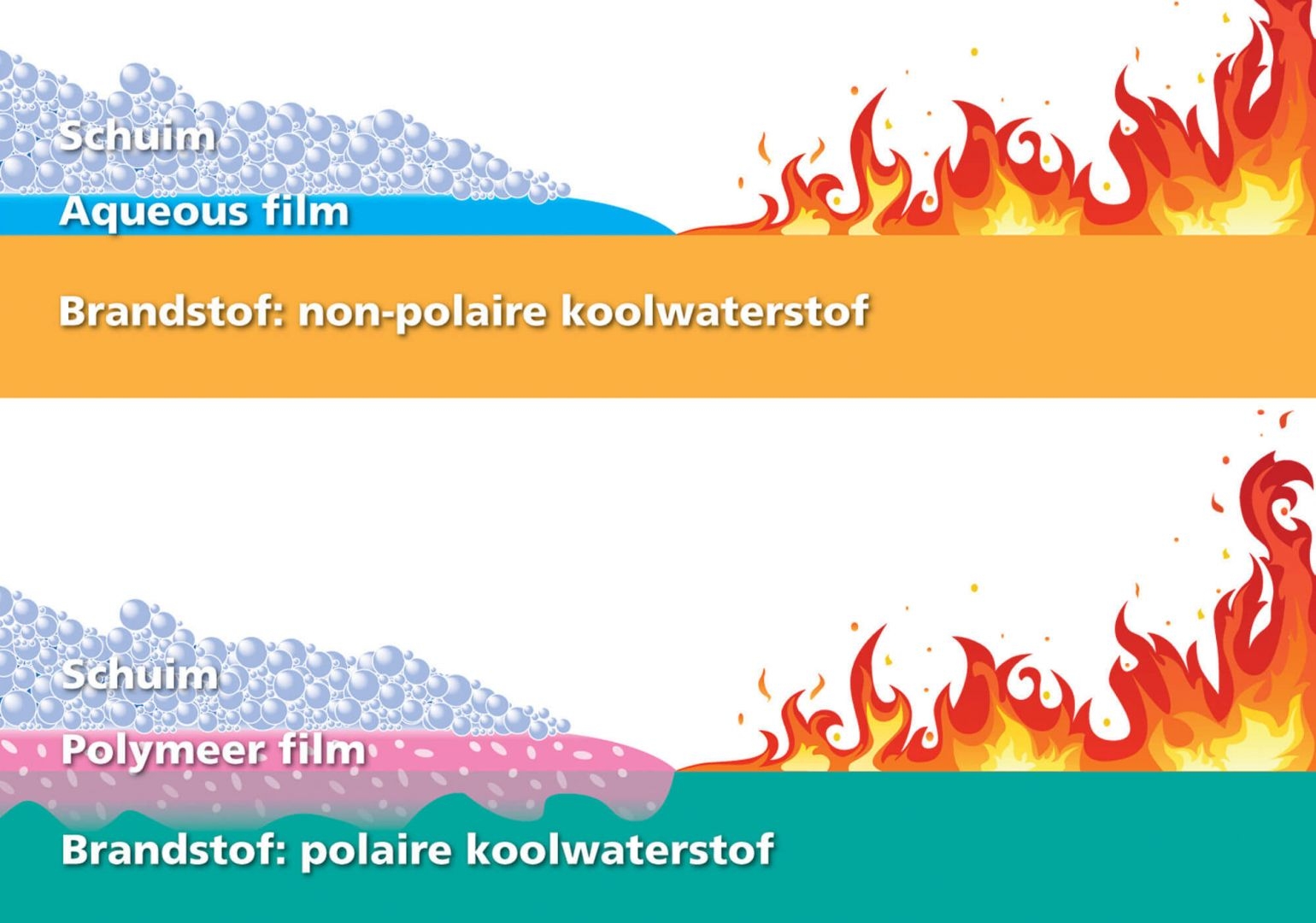
Film forming
Film forming only occurs in fire fighting foams containing fluorine. There are two types of film formation possible.
Aqueous film
Creates a thin layer of water on non-polar liquids after expansion. This film floats away from the foam, has excellent extinguishing properties, and prevents further ignition.
Polymer film
For polar liquids, the use of Aqueous film-forming extinguishing foams is meaningless. This is because polar liquids are lighter than water, the foam will sink. For polar liquids, a foaming agent that forms a polymer film is used. Polymers are lighter than polar liquids and will continue to float. The polymer film floats between the foam and the fuel, separating the alcohol and the foam blanket. A stable polymer film will only be formed if the foam is applied gradually.
Ask us anything
We are happy to help. Fill in the form and we will contact you. Or give us a call +31(0)187 49 35 88. You can also chat with one of our employees.